 您的购物车当前为空
您的购物车当前为空
Ombitasvir
一键复制产品信息别名 奥比他韦, ABT-267
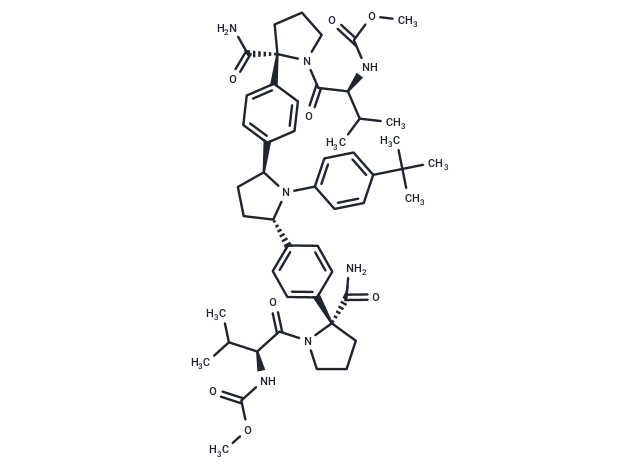
Ombitasvir (ABT-267) 是丙型肝炎病毒非结构蛋白 5A 的口服生物可利用的强效抑制剂。对 HCV 基因型 1 至 5 的 EC50 为 0.82 至 19.3 pM,对基因型 6a 的 EC50 为 366 pM。

为众多的药物研发团队赋能,
让新药发现更简单!
Ombitasvir
一键复制产品信息Ombitasvir (ABT-267) 是丙型肝炎病毒非结构蛋白 5A 的口服生物可利用的强效抑制剂。对 HCV 基因型 1 至 5 的 EC50 为 0.82 至 19.3 pM,对基因型 6a 的 EC50 为 366 pM。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 247 | 现货 | |
| 5 mg | ¥ 745 | 现货 | |
| 10 mg | ¥ 1,160 | 现货 | |
| 25 mg | ¥ 2,380 | 现货 | |
| 50 mg | ¥ 3,480 | 现货 | |
| 100 mg | ¥ 4,960 | 现货 | |
| 200 mg | ¥ 6,860 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 1,130 | 现货 |
Ombitasvir 相关产品
产品介绍
| 产品描述 | Ombitasvir (ABT-267) is an orally bioavailable and potent inhibitor of the hepatitis C virus (HCV) non-structural protein 5A (NS5A).with EC50s of 0.82 to 19.3 pM against HCV genotypes 1 to 5, and 366 pM against genotype 6a |
| 靶点活性 | HCVs 1-5:0.82 pM-19.3 pM(EC50) , HCV 6a:366 pM(EC50) |
| 体内活性 | Ombitasvir was evaluated in vivo in a 3-day monotherapy study in 12 HCV genotype 1-infected patients at 5, 25, 50, or 200 mg dosed once daily. All patients were HCV genotype 1a infected and were without preexisting resistant variants at baseline as determined by clonal sequencing. Decreases in HCV RNA up to 3.1 log10 IU/ml were observed. Resistance-associated variants at position 28, 30, or 93 in NS5A were detected in patient samples 48 hours after the first dose. Clonal sequencing analysis indicated that wild-type virus was largely suppressed by ombitasvir during 3-day monotherapy, and at doses higher than 5 mg, resistant variant M28V was also suppressed. Ombitasvir was well tolerated at all doses, and there were no serious or severe adverse events. These data support clinical development of ombitasvir in combination with inhibitors targeting HCV NS3/4A protease (ABT-450 with ritonavir) and HCV NS5B polymerase (ABT-333, dasabuvir) for the treatment of chronic HCV genotype 1 infection[1]. |
| 动物实验 | The patients in the ombitasvir dose groups were enrolled sequentially, and within each group, patients were randomized (2:1) to either ombitasvir or placebo and treated under nonfasting conditions for 3 days while confined to the study site. The 200-mg dose group received a different formulation with higher bioavailability. Patients who received at least one dose of ombitasvir or placebo were provided the option to receive treatment with pegIFN/RBV for approximately 48 weeks once treatment with ombitasvir was completed. HCV RNA was measured using the Roche COBAS TaqMan HCV Test v2.0 real-time reverse transcriptase PCR assay (with a lower limit of quantification of 25 IU/ml and a lower limit of detection of 10 IU/ml). The virologic response was assessed as HCV RNA decrease from baseline in log10 IU/ml[1]. |
| 别名 | 奥比他韦, ABT-267 |
| 分子量 | 894.11 |
| 分子式 | C50H67N7O8 |
| CAS No. | 1258226-87-7 |
| Smiles | COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@]1(C(N)=O)c1ccc(cc1)[C@@H]1CC[C@H](N1c1ccc(cc1)C(C)(C)C)c1ccc(cc1)[C@]1(CCCN1C(=O)[C@@H](NC(=O)OC)C(C)C)C(N)=O |
| 密度 | 1.223 g/cm3 (Predicted) |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||
| 溶解度信息 | DMSO: 50 mg/mL (55.92 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
| 体内实验配方 | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2 mg/mL (2.24 mM), Sonication is recommended. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | ||||||||||||||||||||||||||||||
溶液配制表 | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||




 很棒
很棒

 |
|
评论内容