 您的购物车当前为空
您的购物车当前为空
(Z)-Orantinib
一键复制产品信息别名 (Z)-奥兰替尼, (Z)-TSU-68, (Z)-TSU68, (Z)-SU-6668, (Z)-SU6668
(Z)-Orantinib ((Z)-SU6668) 是一种高效、选择性、具口服活性的 ATP-竞争性 Flk-1/KDR、PDGFRβ 和 FGFR1 抑制剂(IC₅₀ 分别为 2.1、0.008、1.2 μM)。作为强效抗血管生成与抗肿瘤分子,该化合物可显著诱导既有肿瘤退缩,为研究肿瘤血管生成抑制机制、肿瘤微环境调控及靶向抗癌策略提供关键实验支持,适于实体瘤机制探索及药物筛选。
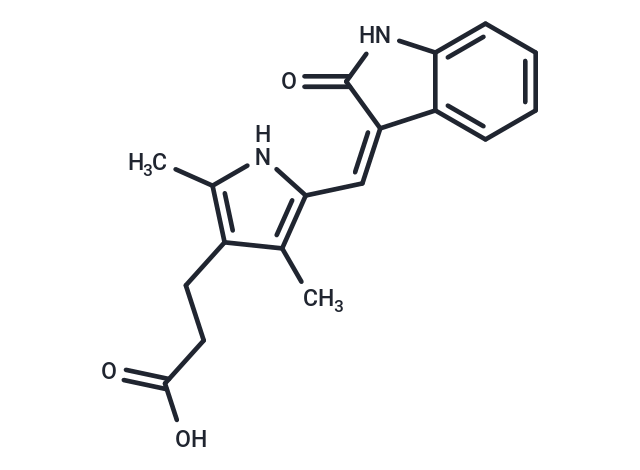
(Z)-Orantinib
一键复制产品信息(Z)-Orantinib ((Z)-SU6668) 是一种高效、选择性、具口服活性的 ATP-竞争性 Flk-1/KDR、PDGFRβ 和 FGFR1 抑制剂(IC₅₀ 分别为 2.1、0.008、1.2 μM)。作为强效抗血管生成与抗肿瘤分子,该化合物可显著诱导既有肿瘤退缩,为研究肿瘤血管生成抑制机制、肿瘤微环境调控及靶向抗癌策略提供关键实验支持,适于实体瘤机制探索及药物筛选。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2 mg | ¥ 194 | 现货 | |
| 5 mg | ¥ 297 | 现货 | |
| 10 mg | ¥ 497 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 547 | 现货 |
(Z)-Orantinib 相关产品
产品介绍
| 产品描述 | (Z)-Orantinib ((Z)-SU6668) is an effective, selective, orally active, ATP-competitive inhibitor of Flk-1/KDR, PDGFRβ, and FGFR1 with IC₅₀ values of 2.1, 0.008, and 1.2 μM, respectively. As a potent anti-angiogenic and anti-tumor compound, (Z)-Orantinib ((Z)-SU6668) induces significant regression in established tumors. (Z)-Orantinib serves as a valuable tool for investigating tumor angiogenesis inhibition mechanisms, tumor microenvironment regulation, and targeted anti-cancer strategy development, providing highly reliable experimental support for exploring solid tumor drug mechanisms and drug screening. |
| 靶点活性 | PDGFRβ:0.008 μM, FLK1/KDR:2.1 μM, FGFR1:1.2 μM |
| 体外活性 | (Z)-Orantinib(5-15 分钟)可抑制Flk-1反式磷酸化、FGFR1反式磷酸化及PDGFR自身磷酸化,其Ki值分别为2.1μM、1.2μM和0.008μM[1]。 |
| 体内活性 | (Z)-Orantinib(4-200 mg/kg,口服,持续21天)对裸鼠体内A431肿瘤的生长表现出剂量依赖性抑制作用 [1]。 |
| 别名 | (Z)-奥兰替尼, (Z)-TSU-68, (Z)-TSU68, (Z)-SU-6668, (Z)-SU6668 |
| 分子量 | 310.35 |
| 分子式 | C18H18N2O3 |
| CAS No. | 210644-62-5 |
| Smiles | C(=C\1/C=2C(NC1=O)=CC=CC2)\C3=C(C)C(CCC(O)=O)=C(C)N3 |
| 颜色 | Orange |
| 物理性状 | Solid |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | DMSO: 40 mg/mL (128.89 mM), Sonication is recommeded. | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||




 很棒
很棒

 |
|
评论内容