 您的购物车当前为空
您的购物车当前为空
Infigratinib mesylate
一键复制产品信息Infigratinib mesylate, also known as, BGJ398 mesylate or NVP-BGJ398 mesylate, is a pan FGFR kinase inhibitor, and is an orally bioavailable pan inhibitor of human fibroblast growth factor receptors (FGFRs) with potential antiangiogenic and antineoplastic activities. pan FGFR kinase inhibitor BGJ398 selectively binds to and inhibits the activities of FGFRs, which may result in the inhibition of tumor angiogenesis and tumor cell proliferation, and the induction of tumor cell death. Infigratinib mesylate was approved in 2021 to treat adults with cholangiocarcinoma whose disease meets certain criteria.
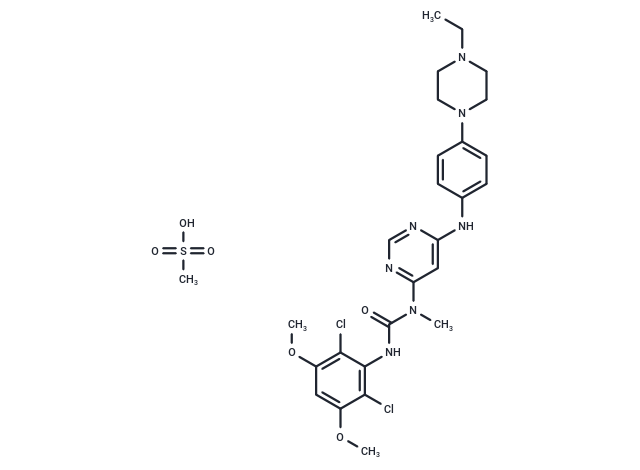
Infigratinib mesylate
一键复制产品信息Infigratinib mesylate, also known as, BGJ398 mesylate or NVP-BGJ398 mesylate, is a pan FGFR kinase inhibitor, and is an orally bioavailable pan inhibitor of human fibroblast growth factor receptors (FGFRs) with potential antiangiogenic and antineoplastic activities. pan FGFR kinase inhibitor BGJ398 selectively binds to and inhibits the activities of FGFRs, which may result in the inhibition of tumor angiogenesis and tumor cell proliferation, and the induction of tumor cell death. Infigratinib mesylate was approved in 2021 to treat adults with cholangiocarcinoma whose disease meets certain criteria.
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25 mg | ¥ 10,600 | 1-2周 | |
| 50 mg | ¥ 13,800 | 1-2周 | |
| 100 mg | ¥ 17,500 | 1-2周 |
Infigratinib mesylate 相关产品
产品介绍
| 产品描述 | Infigratinib mesylate, also known as, BGJ398 mesylate or NVP-BGJ398 mesylate, is a pan FGFR kinase inhibitor, and is an orally bioavailable pan inhibitor of human fibroblast growth factor receptors (FGFRs) with potential antiangiogenic and antineoplastic activities. pan FGFR kinase inhibitor BGJ398 selectively binds to and inhibits the activities of FGFRs, which may result in the inhibition of tumor angiogenesis and tumor cell proliferation, and the induction of tumor cell death. Infigratinib mesylate was approved in 2021 to treat adults with cholangiocarcinoma whose disease meets certain criteria. |
| 分子量 | 656.58 |
| 分子式 | C27H35Cl2N7O6S |
| CAS No. | 1310746-12-3 |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |





 还可以
还可以

 |
|
评论内容