 您的购物车当前为空
您的购物车当前为空
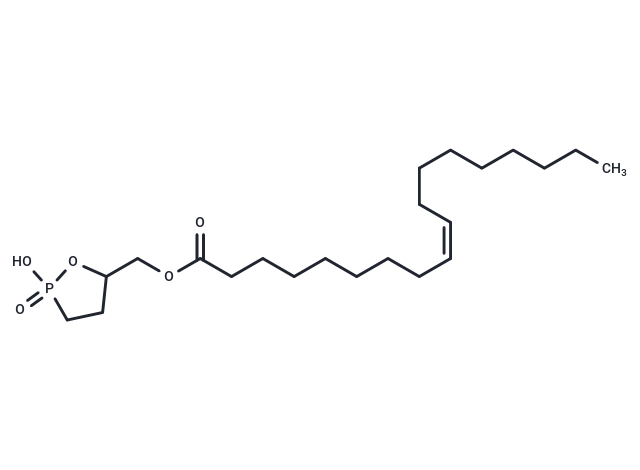
Oleoyl 3-carbacyclic Phosphatidic Acid
一键复制产品信息别名 3-ccPA 18:1
Cyclic Phosphatidic Acids (cPAs) are naturally occurring lysophosphatidic acid (LPA) analogs, characterized by a 5-membered ring formed between the sn-2 hydroxy group and the sn-3 phosphate. Carba-derivatives of cPA (ccPA) modify the sn-2 (2-ccPA) or sn-3 (3-ccPA) linkage, hindering the conversion of cPA into LPA. Oleoyl 3-Carbacyclic Phosphatidic Acid (3-ccPA 18:1) incorporates the 18:1 fatty acid oleate at the sn-1 position on the glycerol backbone, acting as a cyclic LPA analog. This compound, at a concentration of 25 μM, blocks MM1 cells' transcellular migration through mesothelial cell monolayers induced by fetal bovine serum (by 90.1%) or LPA (by 99.9%), without impeding cell proliferation. Additionally, 3-ccPA 18:1, in the 0.1-1.0 μM range, notably suppresses autotaxin, which plays a vital role in various cancer cell behaviors including survival, growth, migration, invasion, and metastasis.
Oleoyl 3-carbacyclic Phosphatidic Acid
一键复制产品信息Cyclic Phosphatidic Acids (cPAs) are naturally occurring lysophosphatidic acid (LPA) analogs, characterized by a 5-membered ring formed between the sn-2 hydroxy group and the sn-3 phosphate. Carba-derivatives of cPA (ccPA) modify the sn-2 (2-ccPA) or sn-3 (3-ccPA) linkage, hindering the conversion of cPA into LPA. Oleoyl 3-Carbacyclic Phosphatidic Acid (3-ccPA 18:1) incorporates the 18:1 fatty acid oleate at the sn-1 position on the glycerol backbone, acting as a cyclic LPA analog. This compound, at a concentration of 25 μM, blocks MM1 cells' transcellular migration through mesothelial cell monolayers induced by fetal bovine serum (by 90.1%) or LPA (by 99.9%), without impeding cell proliferation. Additionally, 3-ccPA 18:1, in the 0.1-1.0 μM range, notably suppresses autotaxin, which plays a vital role in various cancer cell behaviors including survival, growth, migration, invasion, and metastasis.
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mg | 待询 | 8-10周 | |
| 50 mg | 待询 | 8-10周 |
产品介绍
| 产品描述 | Cyclic Phosphatidic Acids (cPAs) are naturally occurring lysophosphatidic acid (LPA) analogs, characterized by a 5-membered ring formed between the sn-2 hydroxy group and the sn-3 phosphate. Carba-derivatives of cPA (ccPA) modify the sn-2 (2-ccPA) or sn-3 (3-ccPA) linkage, hindering the conversion of cPA into LPA. Oleoyl 3-Carbacyclic Phosphatidic Acid (3-ccPA 18:1) incorporates the 18:1 fatty acid oleate at the sn-1 position on the glycerol backbone, acting as a cyclic LPA analog. This compound, at a concentration of 25 μM, blocks MM1 cells' transcellular migration through mesothelial cell monolayers induced by fetal bovine serum (by 90.1%) or LPA (by 99.9%), without impeding cell proliferation. Additionally, 3-ccPA 18:1, in the 0.1-1.0 μM range, notably suppresses autotaxin, which plays a vital role in various cancer cell behaviors including survival, growth, migration, invasion, and metastasis. |
| 别名 | 3-ccPA 18:1 |
| 分子量 | 416.5 |
| 分子式 | C22H41O5P |
| CAS No. | 779333-58-3 |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多





 还可以
还可以
 |
|