- 全部删除
 您的购物车当前为空
您的购物车当前为空
TPST1 Protein, Human, Recombinant (His)
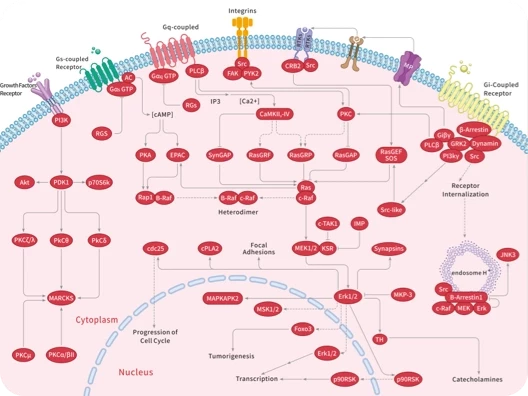
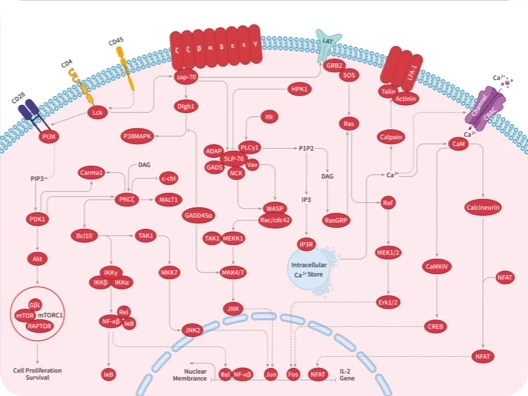
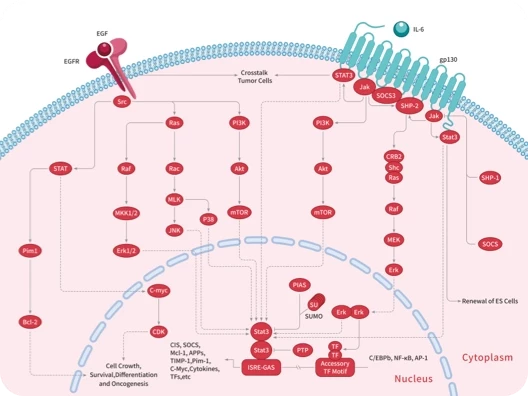
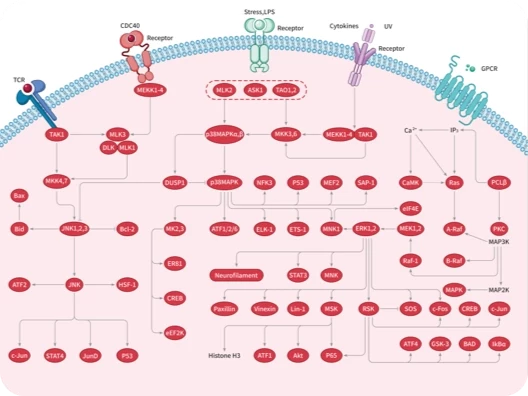
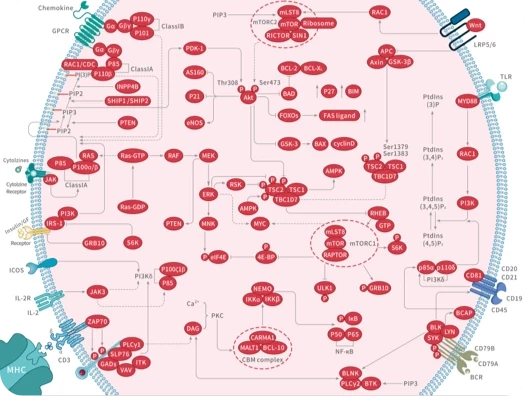
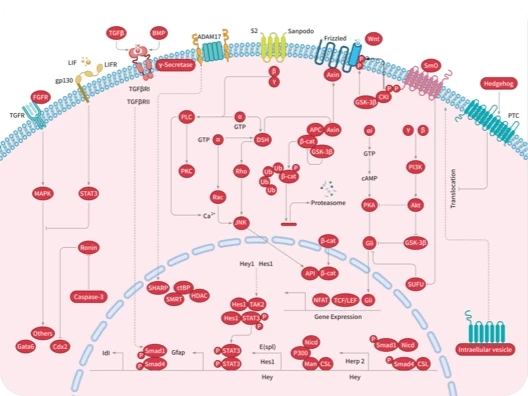
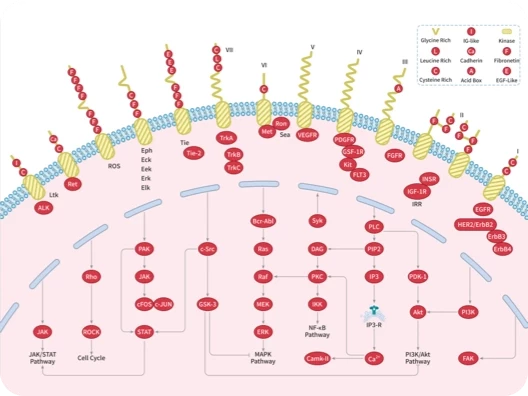
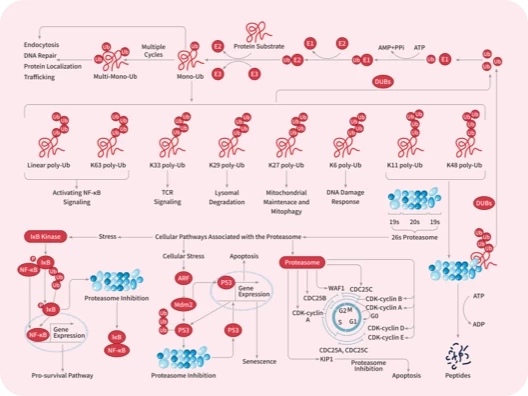
Protein-tyrosine sulfotransferase 1, also known as Tyrosylprotein sulfotransferase 1 and TPST1, is a single-pass type II membrane protein that belongs to the protein sulfotransferase family. Tyrosine O-sulfation is a common posttranslational modification of proteins in all multicellular organisms. This reaction is mediated by a Golgi enzyme activity called tyrosylprotein sulfotransferase (TPST) that catalyzes the transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to tyrosine residues within acidic motifs of polypeptides. Tyrosine O-sulfation has been shown to be important in protein-protein interactions in several systems. Tyrosine sulfation is mediated by one of two Golgi isoenzymes, called tyrosylprotein sulfotransferases (TPST-1 and TPST-2). A relatively small number of proteins are known to undergo tyrosine sulfation, including certain adhesion molecules, G-protein-coupled receptors, coagulation factors, serpins, extracellular matrix proteins, and hormones. TPST1 is a human tyrosylprotein sulfotransferase that uses 3'phosphoadenosine-5'phosphosulfate (PAPS) to transfer the sulfate moiety to proteins predominantly designated for secretion. TPST1 bears N-linked glycosyl residues exclusively at position Asn6 and Asn262. TPST1 and TPST2 have distinct biological roles that may reflect differences in their macromolecular substrate specificity.

TPST1 Protein, Human, Recombinant (His)
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50 μg | ¥ 3,820 | 5日内发货 |
产品信息
| 生物活性 | Activity testing is in progress. It is theoretically active, but we cannot guarantee it. If you require protein activity, we recommend choosing the eukaryotic expression version first. |
| 产品描述 | Protein-tyrosine sulfotransferase 1, also known as Tyrosylprotein sulfotransferase 1 and TPST1, is a single-pass type II membrane protein that belongs to the protein sulfotransferase family. Tyrosine O-sulfation is a common posttranslational modification of proteins in all multicellular organisms. This reaction is mediated by a Golgi enzyme activity called tyrosylprotein sulfotransferase (TPST) that catalyzes the transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to tyrosine residues within acidic motifs of polypeptides. Tyrosine O-sulfation has been shown to be important in protein-protein interactions in several systems. Tyrosine sulfation is mediated by one of two Golgi isoenzymes, called tyrosylprotein sulfotransferases (TPST-1 and TPST-2). A relatively small number of proteins are known to undergo tyrosine sulfation, including certain adhesion molecules, G-protein-coupled receptors, coagulation factors, serpins, extracellular matrix proteins, and hormones. TPST1 is a human tyrosylprotein sulfotransferase that uses 3'phosphoadenosine-5'phosphosulfate (PAPS) to transfer the sulfate moiety to proteins predominantly designated for secretion. TPST1 bears N-linked glycosyl residues exclusively at position Asn6 and Asn262. TPST1 and TPST2 have distinct biological roles that may reflect differences in their macromolecular substrate specificity. |
| 种属 | Human |
| 表达系统 | HEK293 Cells |
| 标签 | N-His |
| 蛋白编号 | O60507 |
| 别名 | tyrosylprotein sulfotransferase 1,TANGO13A |
| 蛋白构建 | A DNA sequence encoding the human TPST1 (NP_003587.1) extracellular domain (Gln 26-Glu 370) was expressed, with a polyhistidine tag at the N-terminus. Predicted N terminal: His |
| 蛋白纯度 | > 80 % as determined by SDS-PAGE |
| 分子量 | 41.7 kDa (predicted); 45-48 kDa (reducing condition, due to glycosylation) |
| 内毒素 | < 1.0 EU/μg of the protein as determined by the LAL method. |
| 缓冲液 | Lyophilized from a solution filtered through a 0.22 μm filter, containing PBS, pH 7.4. Typically, a mixture containing 5% to 8% trehalose, mannitol, and 0.01% Tween 80 is incorporated as a protective agent before lyophilization. |
| 复溶方法 | A Certificate of Analysis (CoA) containing reconstitution instructions is included with the products. Please refer to the CoA for detailed information. |
| 存储 | It is recommended to store recombinant proteins at -20°C to -80°C for future use. Lyophilized powders can be stably stored for over 12 months, while liquid products can be stored for 6-12 months at -80°C. For reconstituted protein solutions, the solution can be stored at -20°C to -80°C for at least 3 months. Please avoid multiple freeze-thaw cycles and store products in aliquots. |
| 运输方式 | In general, Lyophilized powders are shipping with blue ice. |
| 研究背景 | Protein-tyrosine sulfotransferase 1, also known as Tyrosylprotein sulfotransferase 1 and TPST1, is a single-pass type II membrane protein that belongs to the protein sulfotransferase family. Tyrosine O-sulfation is a common posttranslational modification of proteins in all multicellular organisms. This reaction is mediated by a Golgi enzyme activity called tyrosylprotein sulfotransferase (TPST) that catalyzes the transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to tyrosine residues within acidic motifs of polypeptides. Tyrosine O-sulfation has been shown to be important in protein-protein interactions in several systems. Tyrosine sulfation is mediated by one of two Golgi isoenzymes, called tyrosylprotein sulfotransferases (TPST-1 and TPST-2). A relatively small number of proteins are known to undergo tyrosine sulfation, including certain adhesion molecules, G-protein-coupled receptors, coagulation factors, serpins, extracellular matrix proteins, and hormones. TPST1 is a human tyrosylprotein sulfotransferase that uses 3'phosphoadenosine-5'phosphosulfate (PAPS) to transfer the sulfate moiety to proteins predominantly designated for secretion. TPST1 bears N-linked glycosyl residues exclusively at position Asn6 and Asn262. TPST1 and TPST2 have distinct biological roles that may reflect differences in their macromolecular substrate specificity. |