购物车
- 全部删除
 您的购物车当前为空
您的购物车当前为空

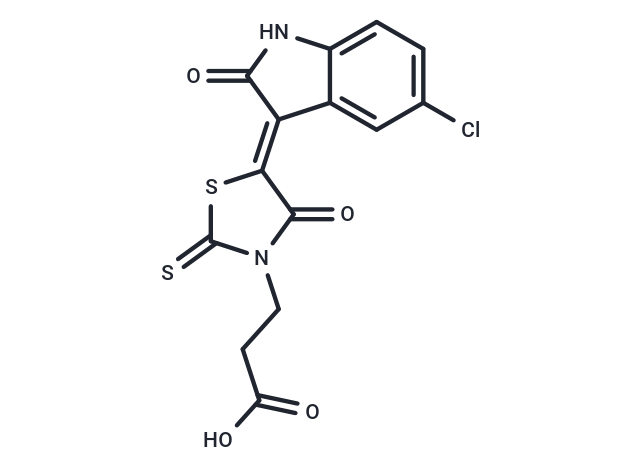
FX1 是一种强效特异性BCL6抑制剂,IC50大约为 35 μM。

为众多的药物研发团队赋能,
让新药发现更简单!
FX1 是一种强效特异性BCL6抑制剂,IC50大约为 35 μM。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 152 | 现货 | |
| 2 mg | ¥ 212 | 现货 | |
| 5 mg | ¥ 373 | 现货 | |
| 10 mg | ¥ 548 | 现货 | |
| 25 mg | ¥ 1,090 | 现货 | |
| 50 mg | ¥ 1,590 | 现货 | |
| 100 mg | ¥ 2,290 | 现货 | |
| 200 mg | ¥ 3,310 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 383 | 现货 |
| 产品描述 | FX1 is an effective and selective BCL6 inhibitor (IC50: 35 μM). |
| 靶点活性 | BCL6 BTB:35 μM |
| 体外活性 | FX1显著降低了SMRT和BCOR对所有三个BCL6靶基因的招募。在BCL6阴性的DLBCL细胞系中,这些位点几乎不含SMRT,且不受FX1影响。经过50 μM FX1处理6小时后,与79-6相比,FX1在定量ChIP试验中打破BCL6与SMRT结合的优越性在DLBCL细胞的头对头比较中变得明显。 |
| 体内活性 | FX1对总B细胞的丰度没有影响。FX1显著消耗生发中心B细胞(GL7+FAS+B220+)。用B220抗体染色揭示了正常的B细胞滤泡结构,而针对生发中心B细胞特异性标记的花生凝集素染色显示GCs的显著丧失。半衰期估计为大约12小时。与对照组相比,用FX1处理的小鼠固定器官的脾脏、肺、胃肠道、肾、心脏、肝脏和骨髓的H&E染色切片未见毒性、炎症或感染迹象。 |
| 细胞实验 | Cell viability is determined with the fluorescent redox dye. Fluorescence is determined for 3 replicates per treatment condition or vehicle with the microplate reader. The drug effect as 100-percentage viability is calculated. Through dose-effect curves the drug concentration that inhibits the growth of cell lines by 50% compared with vehicle (GI50) is determined. Experiments are performed in triplicate. For combination treatments, cells are exposed to a dose curve of each drug alone or their combination in a constant ratio, and cell viability is determined. To compare different schedules of treatments, the cells are treated in triplicate as follows: FX1 and doxorubicin simultaneously and cells treated for 48 hours; FX1 first and 24 hours after doxorubicin is added and treats for an extra 48 hours; doxorubicin first and 24 hours after FX1 is added and treats for an extra 48 hours. Then, the software is used to plot dose-effect curves and calculate the dose-reduction index [1]. |
| 动物实验 | Cell viability is determined with the fluorescent redox dye. Fluorescence is determined for 3 replicates per treatment condition or vehicle with the microplate reader. Cell viability of the drug-treated cells is normalized to their vehicle-treated controls, and the results are expressed as percentage viability. The drug effect as 100-percentage viability is calculated. Through dose-effect curves the drug concentration that inhibits the growth of cell lines by 50% compare with vehicle (GI50) is determined. Experiments are performed in triplicate. For combination treatments, cells are exposed to a dose curve of each drug alone or their combination in constant ratio, and cell viability is determined. To compare different schedules of treatments, the cells are treated in triplicate as follows: FX1 and doxorubicin simultaneously and cells treated for 48 hours; FX1 first and 24 hours after doxorubicin is added and treats for an extra 48 hours; doxorubicin first and 24 hours after FX1 is added and treats for an extra 48 hours. Then, the software is used to plot dose-effect curves and calculate the dose-reduction index[1]. |
| 分子量 | 368.82 |
| 分子式 | C14H9ClN2O4S2 |
| CAS No. | 1426138-42-2 |
| Smiles | OC(=O)CCN1C(=S)S\C(C1=O)=C1/C(=O)Nc2ccc(Cl)cc12 |
| 密度 | no data available |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||
| 溶解度信息 | DMSO: 25 mg/mL (67.79 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
溶液配制表 | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||
评论内容