- 全部删除
 您的购物车当前为空
您的购物车当前为空
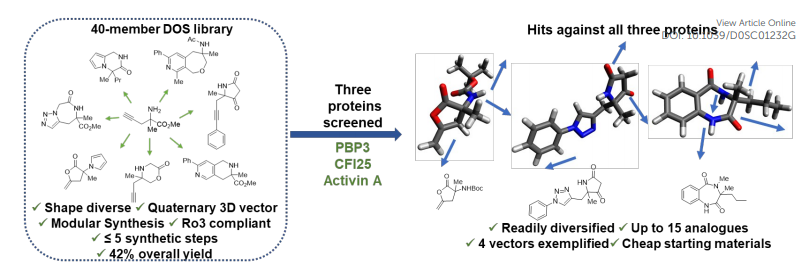
Traditional drug research and development are mainly based on natural active products or screening new drugs from existing compound data, but this method is highly random, blind, and inefficient. Medicinal chemists subsequently developed high-throughput screening (HTS) methods for drug discovery. Many pharmaceutical companies have also established compound libraries containing millions of small molecules and discovered many drug candidates. However, in drug screening with complex targets, HTS has been repeatedly frustrated. It is difficult to screen high-potential compounds, or the screened compounds have high false positives and poor drug-like properties. In this context, Fragment-based drug design (FBDD) came into being. Organic synthesis can transform miniature hits into effective lead compounds. The deficiencies in the current screening of compound libraries usually result in the need for a large amount of synthetic investment to achieve multi-directional fragment growth, which limits the efficiency of the mini-fragment hit modification process. To meet this challenge, we designed a library of highly soluble 3D structural diversity fragments.
The utility of X-ray-based fragment screening and the ability to achieve rapid analog synthesis:





 很棒
很棒